Authors: James Simon Dunn, Jason M Kendall / Editor: Gavin Lloyd / Reviewer: Sarah Hickin-Yacoub, Peter Kilgour, Mehdi Teeli / Codes: A6, O4 / Published: 22/02/2024
Context and Definition
Deep vein thrombosis (DVT) and Pulmonary embolism (PE) form the spectrum of venous thromboembolic disorders (VTE) and are associated with significant mortality and morbidity. PE, whilst it can occur de novo, is most commonly secondary to DVT.
Prevention of VTE by using effective thromboprophylaxis in patients admitted to hospital is a major healthcare priority. This aspect of VTE is not covered in this session.
The early diagnosis of DVT and PE in symptomatic patients significantly reduces adverse outcome.
The initial presentation of a DVT is usually with leg pain and/or swelling. Clearly this has a wide differential diagnosis and presents the clinician with a diagnostic challenge this challenge and the subsequent treatment of DVT is the subject of this session.
What is the definition of a DVT?
DVT is defined as the presence of a blood clot (thrombus) in the deep venous system.
DVT is common. It occurs at a rate of 100-200 per 100,000 of the general population, with 2.5 5% of the population being affected at some point in their lifetime. Up to 50% of DVT patients will suffer long-term consequences including chronic pain, pigmentation or ulceration.1
Anatomy and Pathophysiology
DVT typically presents in the lower limb, although it can also rarely occur in the upper limb.
(i) Anatomy:
The venous anatomy of the leg predisposes itself to the formation of thrombosis: low flow areas such as soleal sinuses, valve pockets and at venous confluences are common sites of clot formation. Clearly the lower limbs are also more prone to the dependent effects of gravity than the upper limbs.
Detectable clot is most commonly found in the distal venous circulation:
- Anterior tibial vein
- Posterior tibial vein
- Peroneal vein
This clot can then propagate proximally into the popliteal, femoral and iliac veins.
(ii) Pathophysiology:
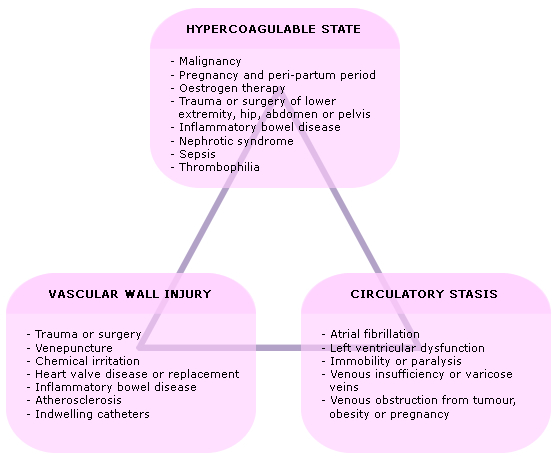
Rudolf Virchow2 reported the relationship between venous stasis, a hypercoaguable state and endothelial vessel wall damage and risk of thrombosis the so called Virchow Triad :
- Venous stasis
- Hypercoagulable state
- Endothelial vessel wall damage
There exists equilibrium between clot formation and clot breakdown; a combination of risk factors may tip this equilibrium in favour of clot formation (thrombosis).
Virchows Triad forms the basis for the pathophysiology of DVT. A combination of the triad of factors is required for thrombosis to form one factor in isolation is not usually sufficient. The presence of each constituent of Virchows triad is, in turn, determined by various factors (Fig 1) which collectively determine risk of DVT in any individual patient.
Fig 1: Risk factors underpinning Virchows triad
The presence and combination of these factors may trigger the pathophysiological process that results in local cytokine production and facilitation of leukocyte adhesion to the endothelium. Whilst the relative contribution of each factor has been long debated, the process reflects the dynamic equilibrium between the pro- and anti-thrombotic tendencies in the deep venous system. The standard treatment strategies for DVT are determined by these factors and are centred on anticoagulation and the prevention of venous stasis.
If the condition goes unrecognised over time, the thrombus subsequently organises and inflammatory cells infiltrate into the clot resulting in intimal thickening. The wall thickens further over time, the likelihood of venous contractility increases, as does the tendency to chronic venous insufficiency causing long term morbidity and increased risk of recurrence.
Regardless of the many described risk factors, only a few have been selected for formal risk stratification in proven clinical studies (see below).
Symptoms and signs are variable at presentation and no single symptom or sign is pathognomonic of DVT. Presenting features are related to the effects of outflow obstruction and local inflammation. However, adaptation of venous collaterals and variable levels of inflammation mean that the degree of thrombus formation (i.e. the clot load ) is frequently unrelated to the clinical findings.
Clinical presentation
Typical features related to the presenting complaint elicited in the history and examination are:
Pain
Pain is present in approx 50% of patients.
Swelling
Swelling/oedema is usually unilateral and the most common sign. Ischaemic change is rare.
Homans sign
Homans sign (pain in the calf upon dorsiflexion of the foot) is unreliable and is often present in calves without DVTs. Anecdotally it may release thrombus into the proximal circulation.
Warmth
Warmth and erythema can occur in an affected calf over the area of the thrombus.
Tenderness
Local tenderness is frequently present, though bears little correlation to the location or extent of thrombus.
Respiratory symptoms
A significant number of patients with a DVT first present with respiratory symptoms: 50% of patients with proven PE are subsequently shown to have a DVT.
Risk factor evaluation:
A careful history searching for relevant risk factors is crucial in determining the most appropriate strategy for investigation; risk factors have been described in the previous section. These are combined with clinical features on presentation to complete a formal risk stratification of patients with suspected DVT (see later section).
There are many other conditions that present with localised pain or oedema in the lower limbs which may be confused with DVT (see Box).
A carefully taken history will be helpful: eg. determine if onset was sudden (musculoskeletal) or gradual (DVT, arterial insufficiency). Examination will help to exclude cellulitis (fever, skin demarcation), or arterial insufficiency (pallor of the leg, absent pulses, sensory changes).
(i) D-Dimer
Following history, evaluation of risk factors and clinical examination, the D-dimer test is the most immediately available investigation used in the diagnostic strategy for DVT in the ED. This test is vulnerable to mis-use (with potential patient harm) if its role is misunderstood.
The test detects fibrin fragments from clot degradation with a high sensitivity when used in appropriately risk-stratified patients with suspected VTE: a negative D-dimer assay in this population results in a 3 month incidence of subsequent VTE of approximately 0.5%.
However, its specificity is poor: D-dimer levels may be raised in any clinical condition in which clot turnover (clot formation and subsequent degradation) is increased.
Such conditions are common and include:
- Infection
- Following trauma
- Following haemorrhage
- Cancer
- Post-surgery
D-dimer assay
The assay also returns increasingly positive with advanced age, irrespective of the potential presence of any of these predisposing factors.
A further important issue is the type of D-dimer assay used: a number of different D-dimer assays are in use throughout the UK, with varying levels of sensitivity and specificity. Each test employs a different monoclonal antibody sensitive to a different part of the D-dimer molecule:
- Latex agglutination assays older tests less accurate and no longer recommended
- enzyme linked immunosorbent assays (ELISA) accurate but time consuming
- rapid ELISA (VIDAS) accurate and rapid version of above
- SimpliRed D-dimer sensitive for proximal DVT, high negative predictive value3
D-dimer levels remain detectable for 7 days after initial clot formation. Testing outside this time may result in a false negative result. The presence of small clot load, particularly in the distal venous system, may provide too small a D-dimer level for a positive result. This may not be of major clinical concern, however, since there is no universal agreement whether isolated distal DVT requires treatment.
The role of D-dimer
It is critical that the result of a D-dimer assay is interpreted in light of the overall clinical picture and with knowledge of the locally used assays characteristics. The assay is always used for its sensitivity (i.e. ability to rule out the diagnosis of DVT) rather than its specificity (i.e. ability to rule it in). This means that, in the appropriate clinical context, it is possible to definitively exclude a diagnosis of DVT and discharge a patient. It is not possible to definitively make the diagnosis: if the D-dimer assay is positive, further investigation is required.
So the crucial question is: in which individual patients is the D-dimer test going to be useful in ruling out a diagnosis of DVT? The answer to this question depends on the pre-test probability of DVT which, in turn, is determined by clinical risk stratification.
The major exception to this principle is pregnant / post-partum women in whom the risk of DVT and/or PE is universally high; there is no role for D-dimers in the investigation of these patients who, if the diagnosis is suspected, require definitive imaging.
Another group of patients that are being increasingly recognised as being at high risk of DVT is the intravenous drug abuser; in particular, they are at risk of septic DVT. This group should also be considered at high risk of DVT and investigated accordingly.
The baseline normal D-dimer level increases with age and in order to avoid false positive results and subsequent over investigation, NICE now suggest an age adjusted D-dimer test threshold should be considered. This means for patients aged 50 and over, their threshold level should equate to either 10x their age, or 5x their age, depending on the assay for D-dimer used locally. This should be confirmed with your laboratory prior to use of this rule.4
all pregnant / post-partum women with suspected DVT or PE are at high risk and need definitive imaging; there is no role for a D-dimer assay
(ii) Clinical risk stratification and use of the Two-level Wells Score
The real value of the D-dimer assay is when it is used appropriately in conjunction with pre-test probability scoring using a risk stratification tool. There are several validated DVT risk stratification tools; the one currently recommended by the National Institute of Clinical Excellence (NICE)4 is the Two-level DVT Wells Score.5
Table: The Two-level Wells DVT Risk Score5
DVT unlikely: Represents low/moderate risk (with a pre-test probability of having a DVT of <15%)5 and this score in combination with a negative D-dimer assay results in a 3 month incidence of DVT of approximately 0.5%. This is low enough to pragmatically exclude the diagnosis and discharge the patient.6,7 A positive D-dimer will require the patient to undergo further imaging.
DVT likely: Represents higher risk (with a pre-test probability of having a DVT of approximately >30%)5 before D-dimer testing. Irrespective of the type of assay used, there is no indication for D-dimer in this group because the post-test probability of a negative assay is still too high to exclude the diagnosis. These patients will all require further imaging.
Learning Bite
Evidence-based risk stratification is critical for appropriate further investigation.
(iii) Imaging
The strategy of good clinical risk stratification and the appropriate use of the D-dimer assay is effective in excluding the diagnosis in approximately 40% of patients presenting with possible DVT.8
However, the only way to definitively confirm the diagnosis of DVT is with further investigation in the form of imaging. Starting long-term anticoagulation without confirmatory imaging is poor clinical practice. Imaging is required in those low risk (DVT unlikely) patients who have a positive D-dimer, and in those at higher risk (DVT likely).
Ultrasonography
Duplex ultrasonography (USS) is now the most commonly used imaging modality in the UK for suspected DVT. The introduction of Doppler flow studies to show real-time imaging aids diagnosis even in the absence of direct visualisation of the clot. Occlusion of the vascular lumen is the major criterion for assessing clot presence but loss of the normal phasic signal from venous blood flow also suggests the presence of occluding clot.
Sensitivity ranges from 97% for proximal DVTs to 73% for distal DVTs.9 Clot more proximal to the inguinal ligament cannot usually be visualised on ultrasonography. Timing of the USS is important: if the USS is not available within 4 hours of being requested then the patient should be administered an interim 24-hour dose of parenteral anticoagulant until the scan is available.
Where persistent diagnostic doubt remains after duplex ultrasonography, or where the result cannot exclude a distal (calf vein) DVT (where there is debate over the benefit of intervention) further action will depend on the initial clinical assessment of risk. If the USS is indeterminate (rather than negative see below) and it is important to make a firm diagnosis (e.g. in the early post-operative phase) then alternative imaging may be required (e.g. venography).
If the USS is negative and the pre-test risk stratification suggested that DVT was likely then current NICE Guidance is for the patient to undergo D-dimer testing at this stage. If the result of this is negative then DVT is excluded; if the D-dimer is positive, then the USS should be repeated approximately 1 week later.4 This pathway is summarised in the Diagnostic algorithm at the end of this section.
Learning bite
If the pre-test probability of DVT is likely and the USS is negative the patient should have a D-dimer to determine the need for a repeat delayed USS.
Other imaging modalities
Other less frequent imaging modalities include:
Impedance plethysmography
- Impedance plethysmography is a non-invasive test that measures small changes in electrical resistance of the calf. These measurements reflect blood volume changes, and can indirectly indicate the presence or absence of venous thrombosis.
- Insensitive for non-occluding thrombus, calf thrombus, or thrombus above the inguinal ligament
MRI
- Approaching venography for sensitivity and specificity
- Highly sensitive for calf thrombus, also for thrombus above the inguinal ligament when CT not practical
- Particular benefit in pregnancy when Doppler flow characteristics change with the gravid uterus
- Limited availability of scanners
CT
- Limited trials report similar results to ultrasonography
- Effective in diagnosis of ileofemoral and more proximal thrombus
- Problems arise with contrast reactions, radiation exposure and presence of metal implants
Venography
- Previously the gold standard, this has now been replaced with non-invasive and less harmful techniques such as ultrasound.
Diagnostic strategy putting it all together (see Fig 2):
The figure below summarises the discussion related to risk stratification, D-dimer usage and USS imaging and demonstrates a NICE-compliant diagnostic pathway for the investigation of patients presenting with suspected DVT.

Increasingly local trusts will have developed an ambulatory care pathway for these patients where any patient with a suspected DVT will be sent to a one-stop clinic with easy access to blood tests, ultrasound and treatment.
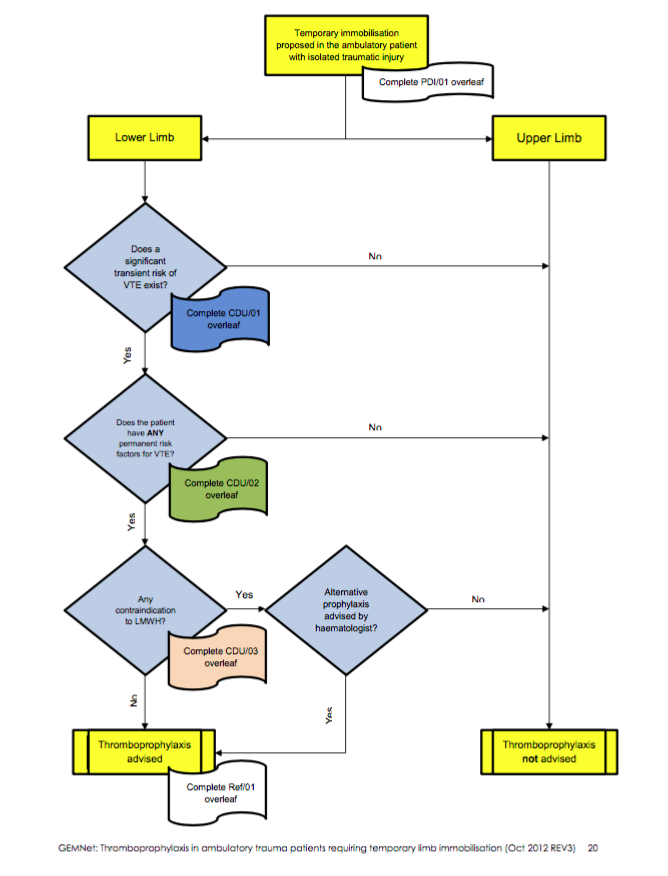
DVT Prevention in the ED
There is increasing evidence to suggest that patients immobilised with a lower limb cast following a fracture are at risk of a DVT. Therefore RCEM have recently released guidelines with an algorithm for risk-stratifying patients.
RCEM Guidelines
Treatment of DVT
The treatment of DVT is aimed at reducing long-term morbidity, preventing recurrence and reducing risk of embolic disease (i.e. PE). The mainstay of therapeutic intervention is anticoagulation. Specifically, in the UK, this is usually achieved with warfarin (oral vitamin K antagonist) in the long term, using low molecular weight heparin (LMWH) or fondaparinux as bridging therapy until the patient has achieved therapeutic anticoagulation with warfarin. Novel oral anticoagulants (NOACs) are being increasingly used and are now recommended as a viable option by new NICE guidelines for definitive treatment for those with confirmed DVT.
There is uncertainty over the role of anticoagulation for isolated distal (below knee) DVT; current NICE Guidance4 recommends anticoagulation for proximal DVT defined as occurring in the popliteal vein or above.
Medium and long term treatment of DVT is the responsibility of GPs or ward based physicians and careful collaboration is required.
Pharmacological treatments
Bridging therapy Heparins and fondaparinux:
Most centres have an initial 4-5 day bridging regime of LMWH injections whilst loading with warfarin. Previous use of infusions of unfractionated heparin has been replaced by LMWH, which does not require daily monitoring and allows out-patient anticoagulation suitable for the vast majority of patients. Unfractionated heparin still has a role in patients with significant renal impairment.
Fondaparinux is now licensed for the treatment of DVT when used in conjunction with warfarin. Trials have compared it with twice daily dosing of enoxaparin to which it is at least comparable.10
Bridging therapy with LMWH or fondaparinux should continue for at least 5 days or until the INR has been >2 for 24 hours, whichever is longer.4
Warfarin
Vitamin K antagonists were previously the mainstay of long-term treatment but are increasingly being replaced by NOACs. Warfarin inhibits the vitamin K-dependent synthesis of biologically active forms of the calcium dependent clotting factors II, VII, IX and X, as well as the regulatory factors protein C, protein S, and protein Z. There is a tendency towards a paradoxical pro-thrombotic state during the first few days of warfarin therapy during which time bridging therapy is required with a LMWH. This resolves from 36 hours onwards as the levels of stored clotting factors diminish.
Warfarin treatment should be continued for a minimum of three months.4 Extended use beyond three months should be considered in patients with an unprovoked (i.e. no clear causative factor) proximal DVT if the risk of recurrence is considered high and there is no major bleeding risk.4
Patients with active cancer should receive long term (6 months) anticoagulation with LMWH (rather than warfarin): achieving therapeutic warfarin levels is difficult in cancer patients due to the increased risk of drug interactions, malnutrition, vomiting, and liver dysfunction in these patients. Moreover, cancer patients are at an increased risk of adverse effects of warfarin therapy. In contrast, low-molecular-weight heparins (LMWHs) are associated with a lower risk of adverse events compared with warfarin in patients with cancer. At 6 months, the relative risks and benefits of continuing anticoagulation should be reassessed.4
Thrombolysis
Thrombolysis of venous clot is an option rarely used in the UK. In trials it is very effective in restoring blood flow and vessel patency and seems to significantly reduce the long-term post-thrombotic complications of DVT. It carries with it a risk of potentially serious bleeding. A recent meta-analysis supports the use of thrombolysis but the dosing and method of administration remains to be determined. Mortality is comparable to those treated with more conventional anti-coagulation.12 Local intravascular injection of thrombolytic into the affected vein is an alternative to systemic thrombolysis.
NICE recommends the use of catheter-directed (i.e. local) thrombolytic therapy for highly selected patients: specifically, those with symptomatic iliofemoral DVT who have symptoms of less than 14 days duration, good functional status, a life expectancy of greater than one year and a low risk of bleeding.
Aspirin
Aspirin is not recommended for treatment of DVT.4

Fig.1 DVT or PE anticoagulation4
Novel oral anticoagulants (NOACs)
These include rivaroxaban and apixaban, which are direct inhibitors of factor Xa. NOACs are becoming popular choices to treat DVT as they have several advantages over warfarin; fewer drug interactions (leading to a more predictable anticoagulant effect) and no need for regular blood tests for monitoring. Treatment regimes approved by NICE include:
Rivaroxban 15 mg twice daily for the first 21 days followed by 20 mg once daily.
Apixaban 10mg twice daily for the first 7 days, followed by 5mg twice daily.
If neither rivaroxaban nor apixaban are deemed suitable, an alternative regime may be LMWH for 5 days followed by dabigatran or edoxaban.
For further guidance on treatment options in those with renal impairment, active cancer or antiphospholipid syndrome, consult the most recent NICE guidelines.4
Mechanical treatments
Compression stockings
Compression stockings, aside from the prevention of DVT, are effective in reducing the effects of post-thrombotic syndrome. Up to 50% of patients who have had a DVT will go on to develop post-thrombotic syndrome in the following 2 years; this is more common in the elderly and in cases of recurrent ipsilateral DVT. Current guidance is that, in addition to anticoagulation, patients are prescribed below-knee graduated compression stockings for at least 2 years, commencing one week following diagnosis.4 Trials suggest this cuts the rate of post-thrombotic syndrome by up to 50%.
Vena caval filters
Trousseau first suggested vena caval filters in 1868. Initial attempts at ligating the vena cava or limiting the diameter had high mortality rates and it wasnt until the 1960s that the first filters were introduced. Since 1970, the Greenfield filter has been in use and reduces the risk of recurrent PE to 4%.13 Whilst not treating the underlying DVT, filters provide a management option in those patients not suitable for anti-coagulation or who have recurrent VTE despite long term warfarin therapy.4
Other treatment issues
Early ambulation poses no risk for clot propagation and is encouraged; it may even reduce the risk of post-thrombotic complications.
Most patients are suitable for outpatient treatment. A recent Cochrane review14 suggested there is no difference in long-term outcome in those patients treated at home compared to those treated in hospital. It would however be prudent to initially admit those with a large proximal clot burden (extending up into the iliac veins), those who are at higher risk of bleeding, those in poor social circumstances, and those that may not be reliable in self-medicating.
Learning bite
The vast majority of patients with suspected or proven DVT can be investigated and managed as an out-patient.
DVT can occur in the upper limb although it is much less common than in the lower limb. It presents with similar symptoms (pain, swelling) and signs (increased circumference, warmth, venous prominence and tenderness) to lower limb DVT.
Risk factors for upper limb DVT include central venous catheters (CVC), malignancy, inherited/acquired thrombophilia, pacemakers and upper limb surgery and/or immobilisation.
The role of D-dimer assay in upper limb DVT is less clear although intuitively it should have similar performance to lower limb DVT. Definitive investigation includes venography or compression ultrasound.
Optimal duration of warfarin therapy is unknown but 3-6 months is associated with low risk of recurrence. Bridging therapy with LMWH is required for at least the first five days as with lower limb DVT.
- DVT is a common condition associated with significant morbidity and mortality
- The diagnostic strategy requires pre-test probability estimation of DVT (level of evidence 1a)
- The combination of pre-test probability scoring and the appropriate use of D-dimer assay will allow exclusion of DVT in about 40% of patients (level of evidence 1a)
- The D-dimer assay is not appropriate in certain patients: this will vary dependant on the type of assay in use and the pre-test risk of DVT (level of evidence 1a)
- Duplex ultrasonography is the current standard imaging modality for the diagnosis of DVT
- Long-term sequelae can be reduced by early and appropriate treatment (level of evidence 1a)
- Scarvelis D, Wells PS. Diagnosis and treatment of deep-vein thrombosis. CMAJ. 2006 Oct 24;175(9):1087-92.
- Encyclopaedia Britannica. Rudolf Virchow. [Accessed Feb 2024].
- Jones S. SimpliRed and diagnosis of deep venous thrombosis. BestBets.org. 2003.
- National Institute for Health and Clinical Excellence (NICE). Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. NG 158, 2020. Updated: 2 August 2023.
- Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003 Sep 25;349(13):1227-35.
- Wells PS, Owen C, et al. Does this patient have deep vein thrombosis? JAMA. 2006 Jan 11;295(2):199-207.
- Schutgens RE, Ackermark P, Haas FJ, et al. Combination of a normal D-dimer concentration and a non-high pretest clinical probability score is a safe strategy to exclude deep venous thrombosis. Circulation. 2003 Feb 4;107(4):593-7.
- Gardiner C, Pennaneach C, et al. An evaluation of rapid D-dimer assays for the exclusion of deep vein thrombosis. Br J Haematol. 2005 Mar;128(6):842-8.
- Goodacre S, Sampson F, et al. Systematic review and meta-analysis of the diagnostic accuracy of ultrasonography for deep vein thrombosis. BMC Med Imaging. 2005 Oct 3;5:6.
- Bller HR, Davidson BL, Decousus H, Gallus A, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004 Jun 1;140(11):867-73.
- Broderick C, Watson L, Armon MP. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No.: CD002783.
- Greenfield LJ, Proctor MC. Twenty-year clinical experience with the Greenfield filter. Cardiovasc Surg. 1995 Apr;3(2):199-205.
- Othieno R, Okpo E, Forster R. Home versus inpatient treatment for deep vein thrombosis. Cochrane Database of Systematic Reviews 2018, Issue 1. Art. No.: CD003076..
- National Institute for Health and Clinical Excellence (NICE). Venous thromboembolism: diagnosis and anticoagulation treatment. 2023.